LOSS OF LOWER LIMB MOTOR EVOKED POTENTIALS AND SPINAL CORD INJURY DURING THE INITIAL EXPOSURE IN SCOLIOSIS SURGERY
Alan D. Legatt,*† Stephen J. Fried,* Terry D. Amaral,‡ Vishal Sarwahi,‡ and Marina Moguilevitch§
Purpose: To report a case of motor evoked potential changes and spinal cord
Methods: Motor evoked potentials to transcranial electrical stimulation were recorded from multiple muscles. Somatosensory evoked potentials to limb nerve stimulation were recorded from the scalp.
Results: Clear motor evoked potentials were initially present in all monitored muscles. The patient was then pharmacologically paralyzed for the initial dissection. More than usual bleeding was encountered during that dissection, prompting transfusion. As the neuromuscular blockade subsided, motor evoked potentials persisted in the hand muscles but disappeared and remained absent in all monitored leg muscles. The spine had not been instrumented. A wake-up test demonstrated paraplegia; the surgery was aborted. There were no adverse somatosensory evoked potential changes. MRI showed an anterior spinal cord infarct.
Conclusions: Copious soft tissue bleeding during the initial dissection might have lowered pressures in critical segmental arteries enough to cause spinal cord infarction through a steal phenomenon. The lack of somatosensory evoked potential changes reflected sparing of the dorsal columns. When neuromuscular blockade is used during the initial soft tissue dissection, motor evoked potentials should be assessed after this, but before spinal instrumentation, to determine whether there had been any spinal cord compromise during the initial dissection.
Key Words: Intraoperative monitoring, Motor evoked potentials, Neuromuscular blockade, Paraplegia, Scoliosis surgery, Spinal cord infarction. (J Clin Neurophysiol 2014;31: e1–e5)
Intraoperative monitoring (IOM) of somatosensory evoked potentials (SEPs) to stimulation of lower limb nerves and of motor evoked potentials (MEPs) recorded from lower limb muscles after transcranial electrical stimulation (TCES) of the brain is used to assess spinal cord function and to detect spinal cord compromise during scoliosis surgery and other spinal operations that place the spinal cord at risk (Nuwer and Packwood, 2008). The wake-up test (Vauzelle et al., 1973) can also be used to assess spinal cord function, although it has risks such as patient self-injury and extubation. Moreover, the wake-up test provides a measurement at a single point in time, whereas SEPs and MEPs can be monitored continuously during the operation, which potentially alerts the surgeons more rapidly when the spinal cord is compromised. A wake-up test can be performed to assess motor function when there are adverse changes in the neurophysiologic IOM data.
Somatosensory evoked potential monitoring antedates MEP monitoring, with the initial reports appearing in the 1970s (Engler et al., 1978; Nash et al., 1972; Nash et al., 1977). Somatosensory evoked potential monitoring has been shown to reduce the incidence of neurologic deficits during scoliosis surgery (Nuwer et al., 1995). However, there are rare cases of patients with new postoperative motor deficits in whom intraoperative SEPs had not shown any significant adverse changes (Ben-David et al., 1987; Ginsburg et al., 1985; Krieger et al., 1992; Zornow et al., 1990). “False-negative” SEP monitoring can occur because the SEPs recorded from the brain are entirely mediated by the dorsal columns within the spinal cord (Cusick et al., 1979; Emerson, 1988), which are perfused by the posterior spinal arteries. The corticospinal tracts lie more anteriorly within the spinal cord and are perfused by the anterior spinal artery. Spinal cord damage during scoliosis surgery is often on a vascular basis (Machida et al., 1988), and an ischemic injury limited to the anterior spinal artery territory, or a mechanical injury to the spinal cord that spares the dorsal columns, may cause a motor deficit without altering the SEPs.
The need to directly monitor the motor tracts within the spinal cord led to the development of MEP monitoring. Motor evoked potentials are typically recorded from limb muscles, such as the tibialis anterior and abductor hallucis muscles in the legs, after TCES of the brain. Complete neuromuscular blockade would eliminate these myogenic responses, and thus would preclude MEP monitoring.
During scoliosis surgery, pharmacologic paralysis may be used during the initial dissection of soft tissue and paraspinal muscles to aid in the dissection. Without neuromuscular blockade, the electrocautery, which is used to perform most of the dissection, would cause prominent muscle contractions. This may make the dissection more difficult, cause increased bleeding, and produce substantial patient movement that could pose a risk to the patient. When the muscular dissection is completed, the neuromuscular blockade is allowed to subside (and may be reversed with agents such as neostigmine) to permit MEP monitoring during the spinal instrumentation and changing the alignment of the vertebral column, which is presumed to be the part of the operation that places the spinal cord at risk. It is usually assumed that the initial dissection does not pose a risk to the spinal cord. However, we report herein a case of a patient with kyphoscoliosis whose spinal cord was injured during the initial dissection.
METHODS
The patient was a 13-year-old girl with myasthenia gravis and severe lordoscoliosis (Fig. 1). At baseline, she was ambulatory without limping. She was being treated with pyridostigmine bromide but had not taken her medication for 2 days before the surgery. At the time of admission, she was ambulatory but was having some difficulty climbing stairs; it was unclear whether this was caused by an exacerbation of her myasthenia, the failure to take the pyridostigmine, or the severe pain from her lordoscoliosis. 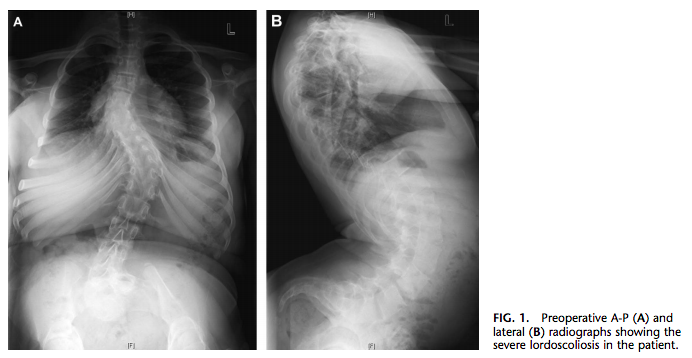
The planned surgical procedure was posterior spinal fusion and instrumentation from T4 to the pelvis to address the significant coronal plane deformity but, more importantly, to address the sagittal plane deformity of excess lumbar lordosis. The patient’s excessive lordosis had caused increased forward pelvic tilt, which caused anterior hip impingement, resulting in both back pain and hip pain.
Neurophysiologic monitoring was performed using a 16- channel XLTek Protektor IOM system (Excel-Tech Ltd, Oakville, ON, Canada). Somatosensory evoked potentials were recorded to stimulation of each posterior tibial nerve at the ankle; CPz–Fpz and CPi–Fpz derivations were used to record the primary cortical SEP component (P37), and an Fpz-inion derivation was used to record the subcortical cervicomedullary SEP component (P31). The stimulus rate was 4.65/s, and the averaging epoch duration was 100 milliseconds. Somatosensory evoked potentials to ulnar nerve stimulation at the wrist (epoch duration: 50 milliseconds) were also monitored as a control for anesthetic effects on cortical SEPs and to detect brachial plexus or peripheral nerve compromise related to arm positioning. The alarm criterion for an SEP was attenuation to 50% of its baseline amplitude.
Single-sweep (unaveraged) MEPs were recorded from needle electrodes in the left and right anal sphincter, in the abductor hallucis, tibialis anterior, and quadriceps femoris muscles in each leg, and in the thenar and hypothenar muscles in each hand. Transcranial electrical stimulation of the brain was performed using brief trains of 500-microsecond duration constant-current stimuli at a rate of 340 per second (2.9-millisecond interstimulus interval within the train) applied between scalp electrode positions C1 and C2 with both stimulus polarities (C1 anode–C2 cathode and C2 anode–C1 cathode). Motor evoked potentials were recorded from a left-to-right anal sphincter derivation, from paired needle electrodes in each monitored leg muscle, and from a thenar-to-hypothenar derivation in each hand, the hand MEP recordings serving as a control for anesthetic and neuromuscular blockade effects on the MEPs recorded from the leg muscles and from the anal sphincter.
The recording epoch for the MEPs was 100 milliseconds in duration. At the beginning of the operation, the TCES stimulus parameters were adjusted to elicit clear MEPs in all of the monitored muscle groups. Because the typical run-to-run variability of myogenic MEPs is much greater than that of SEPs, the alarm criterion for an MEP was attenuation to 10% of its baseline amplitude.
RESULTS
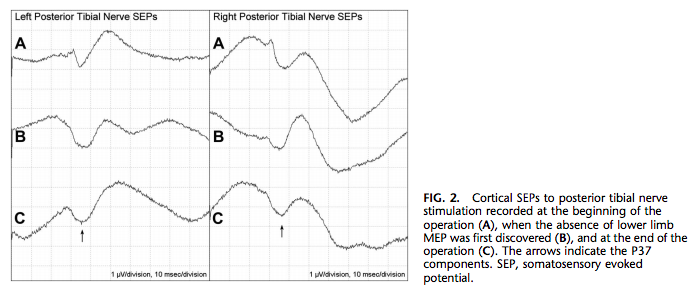
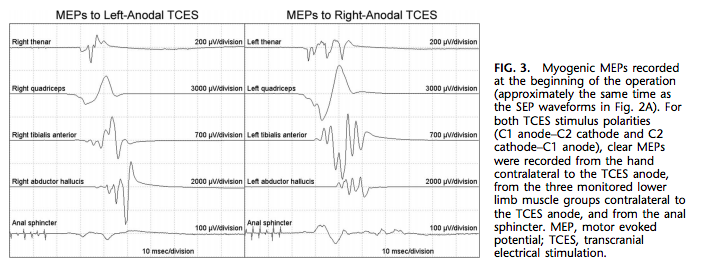
Anesthesia was induced with midazolam, propofol, and sufentanil and maintained with propofol and sufentanil. After the induction of anesthesia and before the initial incision, clear cortical SEPs were recorded after the stimulation of either posterior tibial nerve (Fig. 2A). Also, after titration of the TCES stimulus parameters, clear MEPs were reproducibly recorded from all four monitored limb muscle groups contralateral to the TCES anode and from the anal sphincter after TCES with either stimulus polarity (Fig. 3).
After the baseline IOM data had been obtained, the patient was given rocuronium, 5 mg, to establish paralysis for the initial dissection. Sevoflurane was also transiently administered. Nearly continuous electrocautery use precluded IOM during most of the initial dissection. The amount of bleeding during the initial muscle and soft tissue dissection was more than usual, and the patient’s hematocrit dropped from 36.9 to 24.4, prompting transfusion with 2 units of packed red blood cells. However, she did not become significantly hypotensive.
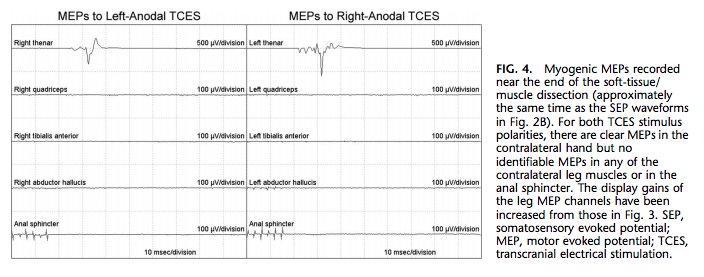
An MEP run recorded approximately 90 minutes after the administration of rocuronium showed very small MEPs in both arms and both legs. However, an MEP run recorded approximately 50 minutes later showed clear MEPs in both hands but no MEPs in either leg or in the anal sphincter (Fig. 4). This finding was reproducible and persisted after the TCES stimulus intensity had been substantially increased. The posterior tibial nerve SEPs were still present (Fig. 2B). The surgeons were notified and performed a wake-up test. The patient moved her arms to command, but there were no leg movements, either spontaneous or to command.
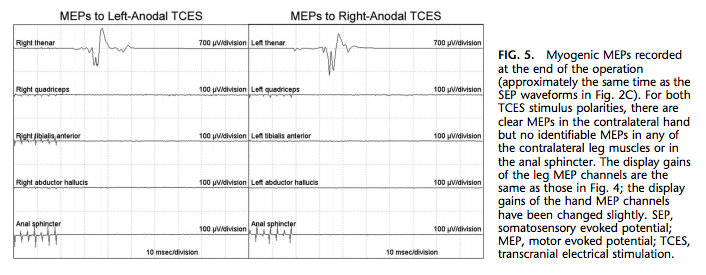
No bone work had been performed, and no screws or instrumentation had been placed. The patient was reanesthetized, and the wound was closed. In the last MEP run recorded at the end of the operation, MEPs were still present in both hands but not in either leg or in the anal sphincter (Fig. 5). Posterior tibial nerve SEPs were still present bilaterally (Fig. 2C).
Postoperatively, the patient moved her arms with good strength and was able to make flicker movements of her feet, but proximal leg muscle strength was 0/5. Light touch, vibration, and position sense were intact bilaterally, including in the toes. Knee jerk and ankle jerk reflexes were 21 bilaterally, and plantar responses were downgoing. MRI showed a new anterior spinal cord abnormality (increased signal within the spinal cord parenchyma) at the T4–T7 level, at the level of the apex of the kyphosis, consistent with an infarct (Fig. 6). There was relative sparing of the posterior portion of the spinal cord. The patient’s leg weakness subsequently improved somewhat with physical therapy.

DISCUSSION
During scoliosis surgery, the spinal cord can be injured by direct mechanical trauma, but in many if not most of the cases in which the patient becomes paraplegic, the injury is thought to be due to compromise of the blood supply to the spinal cord (Machida et al., 1988). The blood supply to the spinal cord is poorly collateralized in the rostral–caudal direction, especially the anterior spinal artery, which may be discontinuous in the mid-thoracic region (Lazorthes et al., 1971). At each segmental level, radicular arteries supply the dorsal and ventral spinal nerve roots bilaterally, but only a small number of these arteries anastomose with the anterior and posterior spinal arteries; the posterior spinal arteries receive more radicular anastomoses than the anterior spinal artery does (Warwick and Williams, 1973).
Thus, perfusion of the lower thoracic spinal cord, especially of the anterior artery territory within that part of the spinal cord, may rely on a small number of radicular arteries, or even on a single one (the artery of Adamkiewicz). Compression of that artery as a result of change in the alignment of the vertebrae can cause spinal cord infarction during scoliosis surgery (Machida et al., 1988). However, the vertebral alignment had not been changed in our patient; in fact, no bone work or instrumentation had been performed at the time the MEPs in the legs were lost.
Our patient had severe postoperative paraparesis, but dorsal column-mediated sensory function was intact, and the MRI was suggestive of an anterior spinal artery territory infarct with relative sparing of the posterior spinal cord (Fig. 6C). This is evidence for an anterior spinal artery territory infarction and an ischemic etiology for her spinal cord injury. Because the SEPs did not change, this is indeed a case of “false-negative” SEP monitoring. However, motor evoked potential monitoring did detect the motor tract compromise.
How did the ischemic injury occur? The dorsal branch of each intercostal artery supplies back muscles and also has a spinal branch that gives rise to the anterior and posterior radicular arteries (Warwick and Williams, 1973). In our patient, the unusually prominent bleeding during the dissection of the back muscles might have lowered the pressure within a critical dorsal branch sufficiently to compromise blood flow through the artery of Adamkiewiczdessentially a vascular steal phenomenon. Significant systemic hypotension would have exacerbated the spinal cord ischemia, but this patient did not become hypotensive during the initial exposure.
It is notable that the injury occurred at the apex of the kyphosis (Fig. 6A), where the spinal cord was pressed against the spine; rostral and caudal to this point, cerebrospinal fluid is visible anterior to the spinal cord (Fig. 6B). The anterior spinal artery itself might have been partially compressed at the apex of the kyphosis, and increased pressure on the anterior spinal cord parenchyma at that site would further lower the spinal cord perfusion pressure (the difference between the pressure within the arterial feeders and the local tissue pressure). The combination of the increased tissue pressure at this level, partial compression of the anterior spinal artery, and lowered pressure within critical radicular arteries due to a vascular steal phenomenon might have lowered perfusion within the anterior spinal cord enough to cause the infarction.
Motor evoked potential monitoring detected the injury and surgery was aborted, and the patient did subsequently recover some motor function in the legs. Because the spinal cord blood supply was already compromised, if the spinal cord injury had not been detected at that point and the surgery had proceeded further, the injury might have been more severe and less reversible. Assessment of MEPs at the end of the soft tissue dissection averted that possibility. When neuromuscular blockade is used for the initial soft tissue dissection during scoliosis surgery, MEPs should be assessed after this, before spinal instrumentation and scoliosis correction, to determine whether there had been any spinal cord compromise during the initial dissection.
REFERENCES
Ben-David B, Haller G, Taylor P. Anterior spinal fusion complicated by paraplegia: a case report of a false-negative somatosensory-evoked potential. Spine 1987;12:536–539.
Cusick JF, Myklebust JB, Larson SJ, Sances A Jr. Spinal cord evaluation by cortical evoked responses. Arch Neurol 1979;36:140–143.
Emerson RG. Anatomic and physiologic bases of posterior tibial nerve somatosensory evoked potentials. Neurol Clin 1988;6:735–749.
Engler GL, Spielholz NI, Bernhard WN, et al. Somatosensory evoked potentials during Harrington instrumentation for scoliosis. J Bone Joint Surg 1978;60:528–532.
Ginsburg HH, Shetter AG, Raudzens PA. Postoperative paraplegia with preserved intraoperative somatosensory evoked potentials: case report. J Neurosurg 1985;63:296–300.
Krieger D, Adams HP, Albert F, et al. Pure motor hemiparesis with stable somatosensory evoked potential monitoring during aneurysm surgery: case report. Neurosurgery 1992;31:145–150.
Lazorthes G, Gouage A, Zadeh JO, et al. Arterial vascularization of the spinal cord. Recent studies of the anastomotic substitution pathways. J Neurosurg 1971;35:253–262.
Machida M, Weinstein SL, Yamada T, et al. Dissociation of muscle action potentials and spinal somatosensory evoked potentials after ischemic damage of spinal cord. Spine 1988;13:1119–1124.
Nash CL Jr, Brodkey JS, Croft TJ. A model for electrical monitoring of spinal cord function in scoliosis patients undergoing correction (Abstract). J Bone Joint Surg 1972;54:197–198.
Nash CL Jr, Lorig RA, Schatzinger LA, Brown RH. Spinal cord monitoring during operative treatment of the spine. Clin Orthop Relat Res 1977;126:100–105.
Nuwer MR, Packwood JW. Monitoring during the surgical treatment of scoliosis. In: Nuwer MR, ed. Intraoperative monitoring of neural function handbook of clinical neurophysiology, volume 8. Amsterdam: Elsevier,
2008:608–617.
Nuwer MR, Dawson EG, Carlson LG, et al. Somatosensory evoked potential spinal cord monitoring reduces neurologic deficits after scoliosis surgery: results of a large multicenter survey. Electroencephalogr Clin Neurophysiol
1995;96:6–11.
Vauzelle C, Stagnara P, Jouvinroux P. Functional monitoring of spinal cord activity during spinal surgery. Clin Orthop Relat Res 1973;93:173–178.
Warwick R, Williams PL. Gray’s anatomy. 35th ed. Philadelphia: W.B. Saunders, 1973.
Zornow MH, Grafe MR, Tybor C, Swenson MR. Preservation of evoked potentials in a case of anterior spinal artery syndrome. Electroencephalogr Clin Neurophysiol 1990;77:137–139.